Electron shell diagram
Table of Contents
Table of Contents
Have you ever wondered how to draw electron shell diagrams? These diagrams are essential to understanding the behavior of atoms in chemistry and physics, but they can be confusing to create.
The process of drawing electron shell diagrams can be frustrating, especially for beginners. Many people struggle with understanding how to properly arrange electrons around an atom’s nucleus and may feel overwhelmed by the number of shells and subshells involved. Additionally, mistakes in electron shell diagrams can lead to incorrect predictions about an atom’s behavior, which can be detrimental in a lab setting.
To draw an electron shell diagram, you must first determine the number of electrons in an atom’s valence shell. Then, you will use this information to create a diagram that shows the electrons in each shell and subshell. This process may seem daunting, but it becomes easier with practice.
In summary, drawing electron shell diagrams is a crucial skill in understanding the behavior of atoms in chemistry and physics. While the process can be challenging, with practice, anyone can learn how to create accurate diagrams.
How to Draw Electron Shell Diagrams: Explained
When I first started learning about electron shell diagrams, I was overwhelmed by the complexity of the diagrams. However, with time and practice, I came to understand the process better, and I want to share my knowledge with you.
To create an electron shell diagram, you will first need to determine the number of electrons in an atom’s valence shell. The valence shell is the outermost shell of an atom, and the number of electrons in this shell determines an atom’s chemical properties. Once you have determined the number of electrons in the valence shell, you can begin to create your diagram.
Start by creating a circle to represent the nucleus of the atom. Then, draw one or more circles around the nucleus, depending on the number of shells in the atom. The first shell, closest to the nucleus, can hold a maximum of two electrons. The second shell can hold up to eight electrons, while the third shell can hold up to 18 electrons.
Next, you will need to determine how many electrons are in each subshell of the atom. Subshells are regions within a shell where electrons with specific energy levels are located. Each subshell can hold a specific number of electrons, and the order of subshells is determined by the energy of their electrons.
Once you have determined the number of electrons in each subshell, draw arrows to represent the electrons. For example, if an atom has one electron in the first subshell and three electrons in the second subshell, you would draw one arrow in the first subshell and three arrows in the second subshell. Remember to follow the rules for placing electrons in subshells, such as the Aufbau principle and the Pauli exclusion principle.
Tips for Drawing Electron Shell Diagrams
One of the best ways to improve your ability to draw electron shell diagrams is to practice. Start by drawing diagrams for simple atoms with fewer electrons, such as hydrogen or helium, and then move on to more complex diagrams. It may also be helpful to work with a partner or tutor who can provide feedback on your diagrams.
When creating your diagrams, remember the importance of accuracy. Mistakes in electron shell diagrams can lead to incorrect predictions about an atom’s behavior, so take the time to double-check your work. Also, be sure to follow the rules for placing electrons in subshells to ensure that your diagrams are accurate.
The Benefits of Learning How to Draw Electron Shell Diagrams
The ability to draw electron shell diagrams is essential for anyone studying chemistry or physics. These diagrams provide a visual representation of an atom’s electron configuration, which allows scientists to predict how an atom will react in different chemical and physical environments. By understanding electron shell diagrams, you can gain a deeper understanding of the behavior of atoms and their interactions with other elements.
Common Mistakes to Avoid When Drawing Electron Shell Diagrams
One of the most common mistakes people make when drawing electron shell diagrams is forgetting to follow the rules for placing electrons in subshells. Remember to follow the Aufbau principle, which states that electrons fill lower-energy subshells before higher-energy subshells. Additionally, be sure to follow the Pauli exclusion principle, which states that no two electrons in an atom can have the same set of quantum numbers.
Question and Answer
Q: Do electron shell diagrams represent the actual physical structure of an atom?
A: No, electron shell diagrams are diagrams used to represent the electron configuration of an atom. They do not depict the actual physical structure of an atom.
Q: Can electron shell diagrams be used to predict an atom’s behavior in different chemical environments?
A: Yes, electron shell diagrams allow scientists to predict how an atom will behave in different chemical environments based on its electron configuration.
Q: How can I improve my ability to draw electron shell diagrams?
A: Practice is key to improving your ability to draw electron shell diagrams. Start with simple atoms and work your way up to more complex diagrams. It may also be helpful to work with a partner or tutor who can provide feedback on your diagrams.
Q: What are some common mistakes to avoid when drawing electron shell diagrams?
A: Some common mistakes to avoid include forgetting the rules for placing electrons in subshells, incorrectly representing the number of electrons in a subshell, and failing to follow the Pauli exclusion principle.
Conclusion of How to Draw Electron Shell Diagrams
Drawing electron shell diagrams can be challenging, but it is essential for anyone studying chemistry or physics. By understanding how to create accurate diagrams, you can gain a deeper understanding of an atom’s behavior and interactions with other elements. Remember to take your time, practice, and follow the rules for placing electrons in subshells to ensure that your diagrams are accurate.
Gallery
What The Electron Isn’t – A Matter Of Physics

Photo Credit by: bing.com / nucleus teachoo fuse kindpng mechanics
A-Level Chemistry: 1.6.1c Draw Electron Configuration Diagrams Of
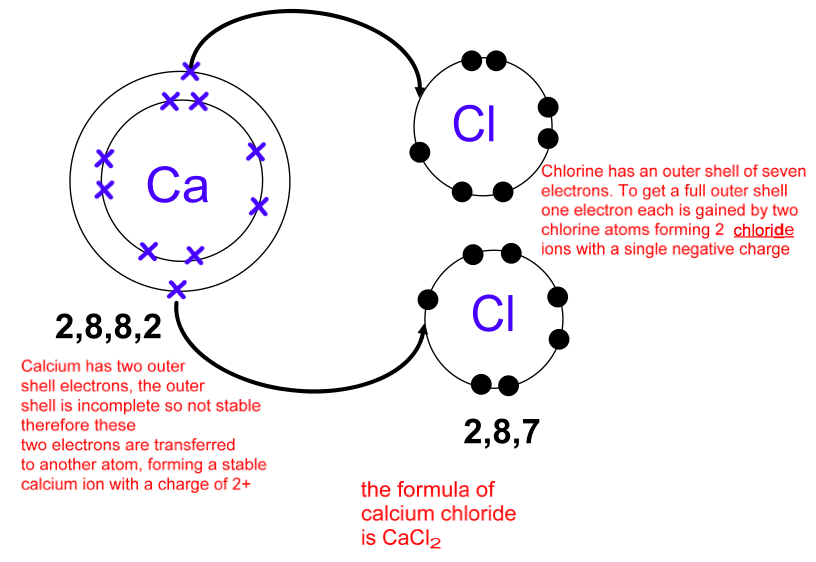
Photo Credit by: bing.com / covalent electrons configuration electron bond draw chemistry atom diagrams simple bonds anions cations level molecules crosses represent when dative ions
Reading: Electrons | Biology I
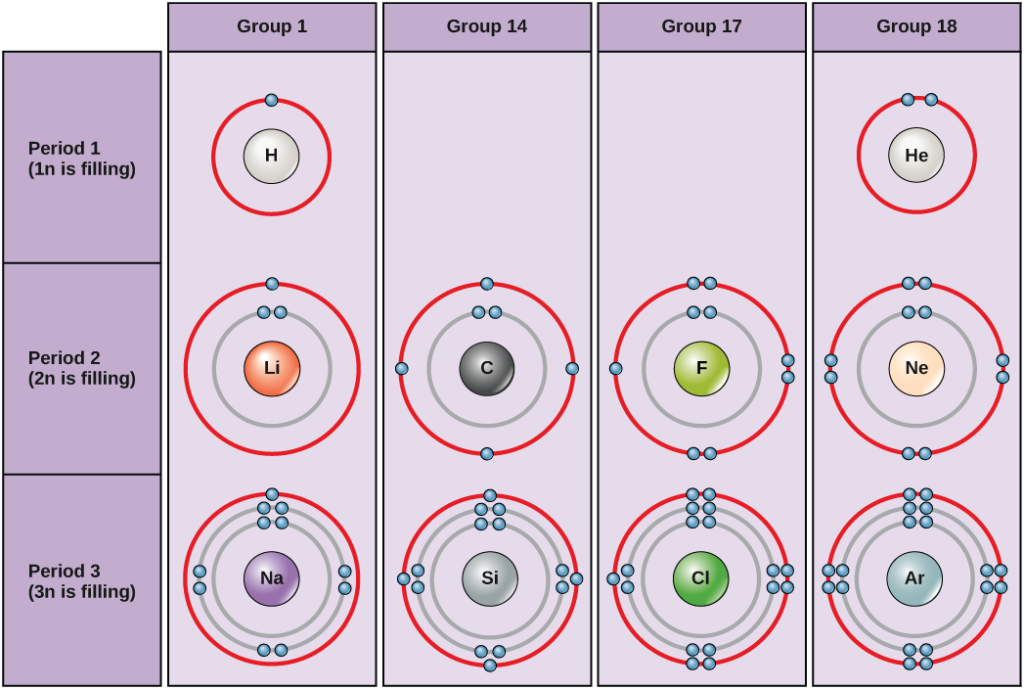
Photo Credit by: bing.com / electrons bohr carbon hydrogen helium sodium diagrams silicon chlorine electron lithium argon stable figure neon fluorine group elements shell configuration
Octet Rule: Why Are Atoms With 8 Valence Electrons So Stable?
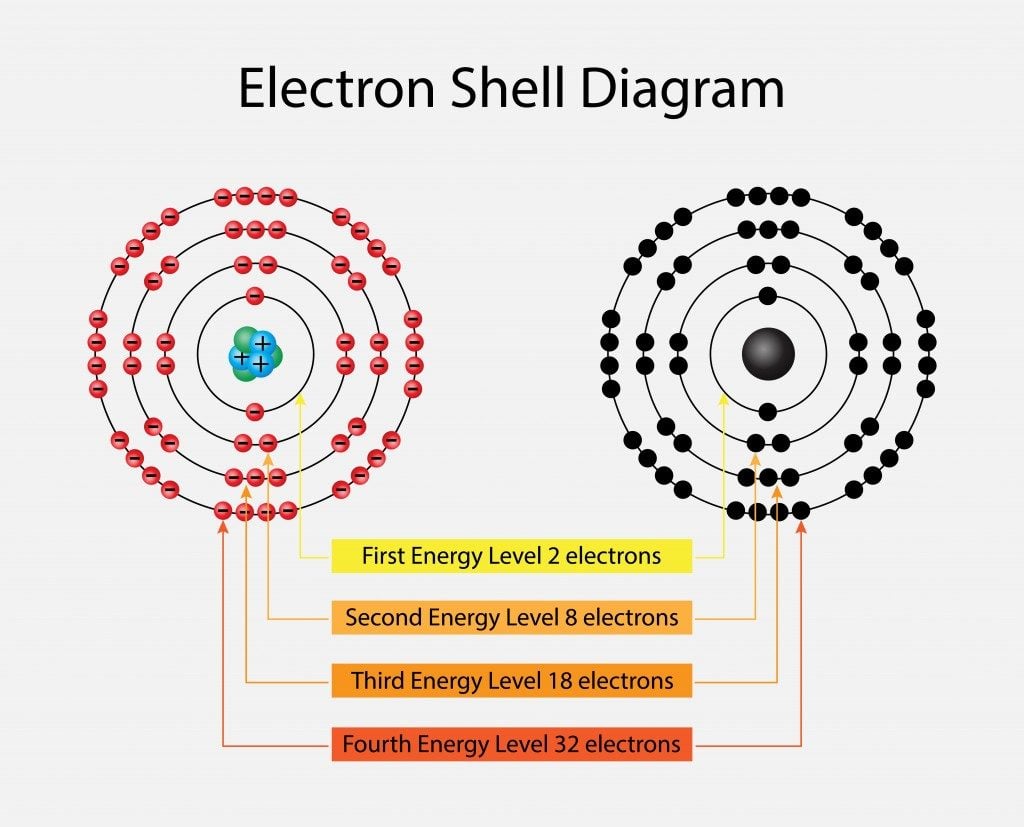
Photo Credit by: bing.com / electron electrons valence atoms stable configuration octet metals orbitals nucleus scienceabc
Electron Shell Diagram | Electrons, Science Projects, Shells

Photo Credit by: bing.com / electron tutorcircle






